Igniting a spark for learning in primary school students
Discovering the learning power of the periodic table
A FLEET visit building better understanding of electricity and atomic structure uncovered several lessons in the role of hands-on investigation in building student understanding
A cooperative FLEET project with a Melbourne primary school taught students to think about atoms, electricity, conductors and insulators, with an 85-student Year 6 pilot workshop testing success in building understanding of atomic structure and electricity, and promoting critical thinking about electricity, society’s application of it, and more broadly the value of research.
Built-in evaluation processes indicated those objectives were met, but also revealed that students’ hands-on investigations, including examining a periodic table, building a model atom, and building different electrical circuits, were crucial to that improved understanding.
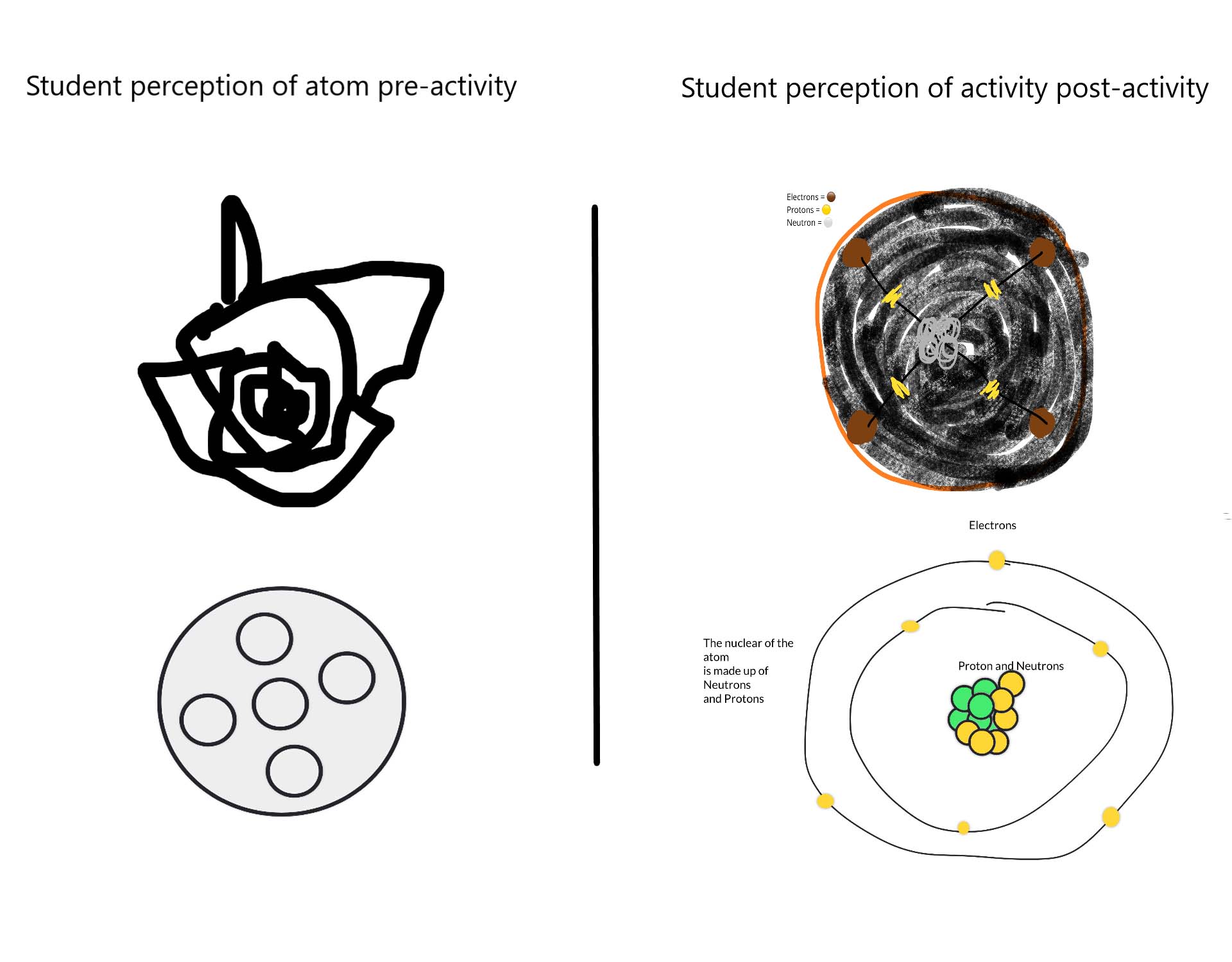
Students were asked to draw their ideas about the structure of an atom.
Students were asked to consider how electricity has changed the world and imagine how they would feel if we did not have electricity: “Without electricity, it would be hard to communicate to family that don’t live near you.” “There would be no toasters no baths no lights no iPads or PlayStation.” Coping with Covid without electricity would be bad: “We would feel sad and lonely in lockdown without power.”
Introducing the atom and the power of the periodic table
Students were asked to draw an atom, both before and after an in-class discussion and hands-on exercise about atomic structure. Students’ initial ideas about atoms (often an indistinct blob with no distinct nucleus) were significantly improved by using the periodic table to select an atom to construct a physical model.
The model atom exercise not only allowed conceptualisation of atomic structure. It also provoked the realisation that atoms come with different sizes and properties to make up all the different elements of the universe.
There was a realisation that the type of atoms determines what they can feel, see, touch or smell: students commented they’d learned that there are different types of atoms, and that “the different atoms are determined by the number of protons, neutrons and electrons”.
“The power of the periodic table as a learning tool in this context was unexpected!” says FLEET outreach coordinator Jason Major.
Building better circuits
Base case understanding: Less than half the students managed a correct circuit on their first attempt, with multiple wires being a common misunderstanding.
Building on their new knowledge of atoms and charged particles, students examined circuits, electrical conductors and insulators, and resistance. After an initial brainstorming session, students drew an electrical circuit, establishing a baseline understanding to evaluate subsequent learning.
After in-depth discussion, and hands-on circuit construction, nearly all students were able to describe a correct circuit. Most understood which way electrons flowed through a circuit, and the charged particles’ role in the flow of charge and electricity (“Electrons only produce electricity when moving”) and practical considerations were also learned (“When you power something up, the alligator clips get hot”).
Considering the value of research
As an example to get students thinking about societal value of science, the class talked about FLEET’s research mission to develop low-energy electronics, within the context of the computational and energy requirement of digital technologies.
Students thought critically about the value of such research, with a high focus on societal challenges such as sustainability, climate change and making the world better: “We would waste less electricity” and “It would help reduce climate change”. They became aware of the value of reducing energy consumption and the value of having energy-efficient technologies to help achieve that goal.
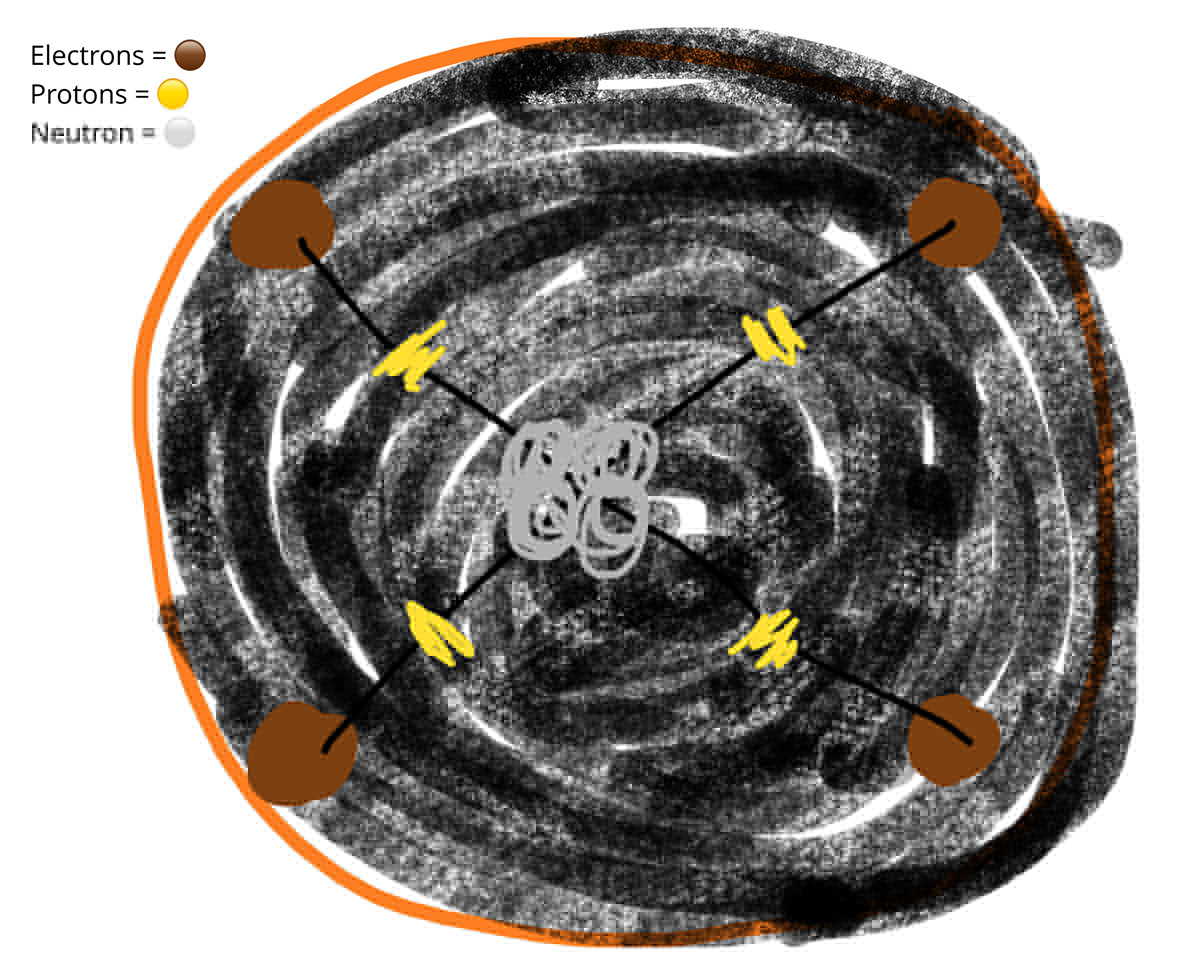
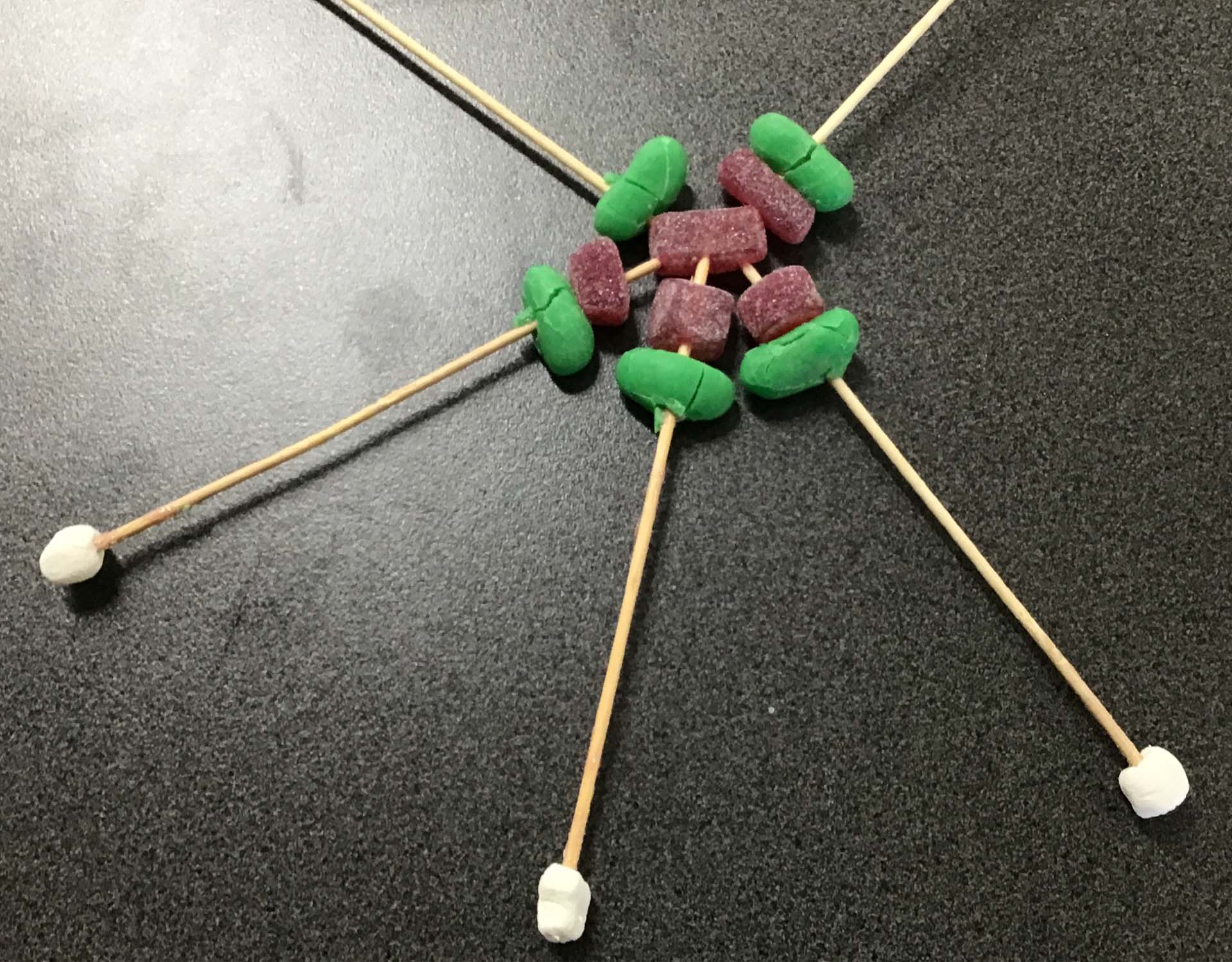
Students' understanding of atomic structure improved markedly on initial concepts (left/top), after being coached to construct a 'lolly model' (right/bottom)